Physical Properties
Targets, pieces, & powder
Chemical Properties
99.8% to 99.99%
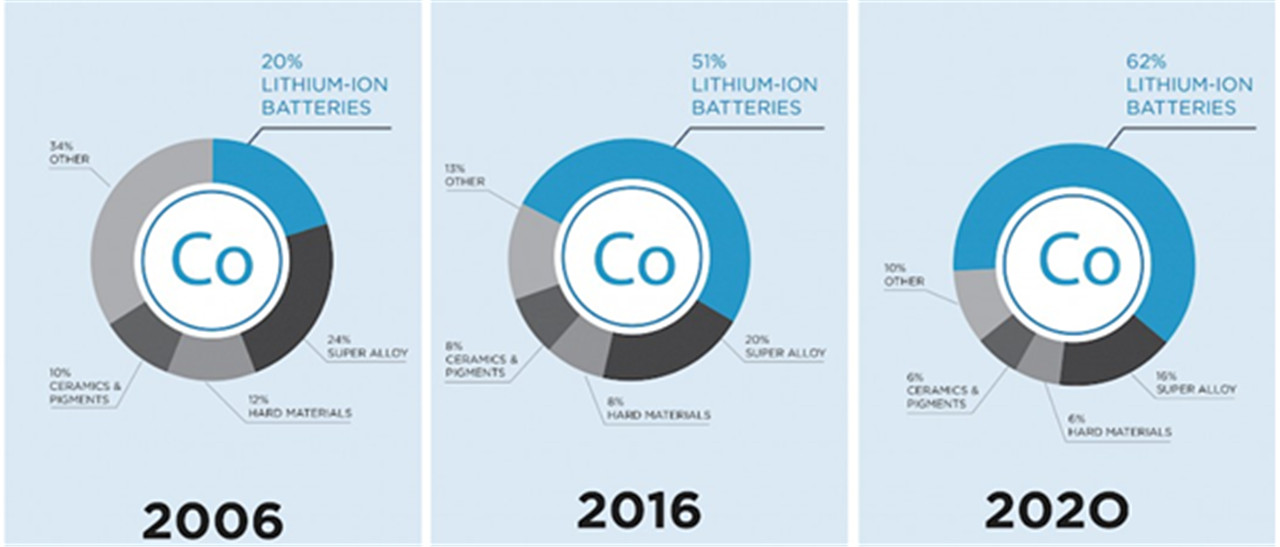
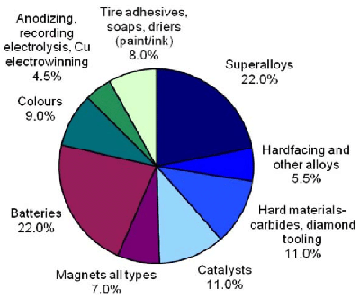
This versatile metal has consolidated its position in traditional areas, such as superalloys, and has found greater use in some newer applications, such as in rechargeable batteries
Alloys-
Cobalt-based superalloys consume most of the produced cobalt. The temperature stability of these alloys makes them suitable for use in turbine blades for gas turbines and jet aircraft engines, though nickel-based single crystal alloys surpass them in this regard. Cobalt-based alloys are also corrosion and wear-resistant. Special cobalt-chromium-molybdenum alloys are used for prosthetic parts such as hip and knee replacements. Cobalt alloys are also used for dental prosthetics, where they are useful to avoid allergies to nickel. Some high speed steels also use cobalt to increase heat and wear-resistance. The special alloys of aluminium, nickel, cobalt and iron, known as Alnico, and of samarium and cobalt (samarium-cobalt magnet) are used in permanent magnets.
Batteries-
Lithium cobalt oxide (LiCoO2) is widely used in Lithium ion battery electrodes. Nickel-cadmium (NiCd) and nickel metal hydride (NiMH) batteries also contain significant amounts of cobalt.
Catalyst-
Several cobalt compounds are used in chemical reactions as catalysts. Cobalt acetate is used for the production of terephthalic acid as well as dimethyl terephthalic acid, which are key compounds in the production of Polyethylene terephthalate. The steam reforming and hydrodesulfuration for the production of petroleum, which uses mixed cobalt molybdenum aluminium oxides as a catalyst, is another important application. Cobalt and its compounds, especially cobalt carboxylates (known as cobalt soaps), are good oxidation catalysts. They are used in paints, varnishes, and inks as drying agents through the oxidation of certain compounds. The same carboxylates are used to improve the adhesion of the steel to rubber in steel-belted radial tires.
Pigments and coloring-
Before the 19th century, the predominant use of cobalt was as pigment. Since the midage the production of smalt, a blue colored glass was known. Smalt is produced by melting a mixture of the roasted mineral smaltite, quartz and potassium carbonate, yielding a dark blue silicate glass which is grinded after the production. Smalt was widely used for the coloration of glass and as pigment for paintings. In 1780 Sven Rinman discovered cobalt green and in 1802 Louis Jacques Thénard discovered cobalt blue. The two colors cobalt blue, a cobalt aluminate, and cobalt green, a mixture of cobalt(II) oxide and zinc oxide, were used as pigments for paintings due to their superior stability. Cobalt has been used to color glass since the Bronze Age.
Description
A brittle, hard metal, resembling iron and nickel in appearance, cobalt has a magnetic permeability approximately two thirds that of iron. It is frequently obtained as a byproduct of nickel, silver, lead, copper, and iron ores and is present in meteorites.
Cobalt is often alloyed with other metals due to its unusual magnetic strength and is used in electroplating because of its appearance, hardness and resistance to oxidation.
Chemical Name: Cobalt
Chemical Formula: Co
Packaging: Drums
Synonyms
Co, cobalt powder, cobalt nanopowder, cobalt metal pieces, cobalt slug, cobalt metal targets, cobalt blue, metallic cobalt, cobalt wire, cobalt rod, CAS# 7440-48-4
Classification
Cobalt (Co) Metal TSCA (SARA Title III) Status: Listed. For further information please contact
UrbanMines Tech. Limited by mail: marketing@urbanmines.com
Cobalt (Co) Metal Chemical Abstract Service Number: CAS# 7440-48-4
Cobalt (Co) Metal UN Number: 3089