What are Rare-Earths?
Rare earths, also known as rare earth elements, refer to 17 elements on the periodic table that include the lanthanide series from atomic numbers 57, lanthanum (La) to 71, lutetium (Lu), plus scandium (Sc) and yttrium (Y).
From the name, one may assume that these are “rare,” but in terms of minable years (the ratio of confirmed reserves to annual production) and their density within the earth’s crust, they are actually more abundant than led or zinc.
By effectively using rare earths, one can expect dramatic changes to conventional technology; changes such as technological innovation through newfound functionality, improvements to durability in structural materials and improved energy efficiency for electronic machines and equipment.
About Rare-Earth Oxides
The Rare-Earth Oxides Group is sometimes referred to as just the Rare Earths or sometimes as REO. Some rare earth metals have found more down to earth applications in metallurgy, ceramics, glass making, dyes, lasers, televisions and other electrical components. The importance of rare earth metals is most certainly on the rise. It has to be taken into account, as well, that most of the rare earth-containing materials with industrial applications are either oxides, or they are obtained from oxides.
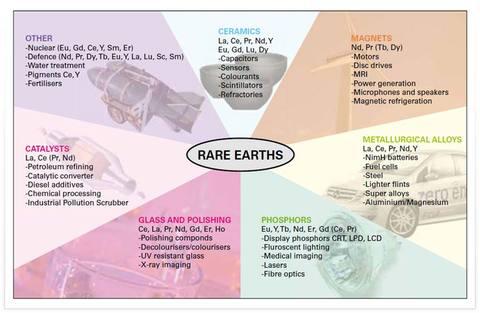
Regarding the bulk and mature industry applications of rare earth oxides, their use in catalysts formulations (such as in three way automotive catalysis), in glass-related industries (glass making, decolouring or colouring, glass polishing and other related applications), and permanent magnets manufacturing account for almost 70% of rare earth oxides usage. Other important industrial applications concern the metallurgy industry (used as additives in Fe or Al metal alloys), ceramics (specially in the case of Y), lighting-related applications (in the form of phosphors), as battery alloy components, or in solid oxide fuel cells, amongst others. Additionally, but not less important, there are lower scale applications, such as biomedical uses of nanoparticulated systems containing rare earth oxides for cancer treatment or as tumoral detection markers, or as sunscreens cosmetics for skin protection.
About Rare-Earth Compounds
High purity Rare-Earth Compounds are produced from ores by the following method: physical concentration (e.g., flotation), leaching, solution purification by solvent extraction, rare earth separation by solvent extraction, individual rare earth compound precipitation. Finally these compounds form marketable carbonate, hydroxide, phosphates and fluorides.
About 40% of rare earth production is used in metallic form—for making magnets, battery electrodes, and alloys. Metals are made from the above compounds by high-temperature fused salt electrowinning and high temperature reduction with metallic reductants, for example, calcium or lanthanum.
Rare earths are mainly used in the following:
● Magnets (up to 100 magnets per new automobile)
● Catalysts (automobile emission and petroleum cracking)
● Glass polishing powders for television screens and glass data storage disks
● Rechargeable batteries (especially for hybrid cars)
● Photonics (luminescence, fluorescence and light amplification devices)
● Magnets and photonics are expected to grow significantly over the next few years
UrbanMines supplies a comprehensive catalog of high purity and ultra high purity compounds. The importance of Rare Earth Compounds grows strongly in many key technologies and they are irreplaceable in many products and production processes. We supply Rare Earth Compounds in different grades according to individual customer requirements, which serves as valuable raw materials in various industries.
What are Rare-Earths generally used in?
The first industrial usage of rare earths was for the flint in lighters. At that time, the technology for separation and refinement had not been developed, so a mixture of multiple rare earth and salt elements or unaltered misch metal (alloy) was used.
From the 1960’s, separation and refinement became possible and the properties contained within each rare earth were made evident. For their industrialization, they were first applied as cathode-ray tube phosphors for colored TVs and on high refractive camera lenses. They have gone on to contribute to reducing the size and weight of computers, digital cameras, audio devices and more through their use in high performance permanent magnets and rechargeable batteries.
In recent years, they have been gaining attention as a raw material for hydrogen-absorbing alloys and magnetostriction alloys.






